Is a particle a wave? Is a mango a line? Some things can never be known for certain.
Quantum Physics has always been that one mysterious theory that has been really difficult to understand. Richard Feynman, a noble prize winner in this field once said:
“If you think you understand quantum mechanics, you don’t understand quantum mechanics”
Which is kind of demoralizing, ’cause if he didn’t understand it, what chance do we have? I think it has been so difficult to understand because almost everything in the Quantum Realm, defies common sense, which makes it really hard to comprehend the strange phenomenons taking place there.
Most of us have a general idea of what the whole thing is about. But for those of my friends that don’t, theoretical physics is mainly divided into two parts: General Relativity and Quantum Mechanics.
General Relativity describes all the stuff that happens on a large measurable level (though it does get a bit too large at times). Things like space, the planets, the stars, and so on.
Quantum Mechanics, on the other hand, covers the small, at the micro level. It discusses how electrons behave, how light moves, and the behavior of all those tinier-than-tiny micro-microscopic particles that we almost don’t know about.
So what exactly is Quantum Physics? Why do we need a separate theory for this microscopic world? After all, these are the things that make up the entire universe. In theory, our theories should work just fine there as anywhere else.
In reality, however, they don’t.
When you shrink an object to the size of an atom, gravity and light work very, very differently. And many new forces such as the weak and strong nuclear force come into play, that have absolutely no effects up in the visible world.
Gravity is a relatively weak force, which only seems stronger because it can operate over large distances. When things are really close together, like in the middle of an atom, then the other forces dominate. (And yes, the ‘weak force’ is stronger).
Hold on a moment — do you know what an atom is? That’s an idea that might need changing.
Okay, so I’m assuming that you have a basic idea of what “atomic particles” — meaning electrons, protons, and the like — are. Tiny objects that sit in the core of the atom or spin around it. Right?
I’m guessing you studied the Bohr’s Model, which looks somewhat like this:

According to this model, the negatively-charged electrons are circling around a positively charged nucleus.
Bohr thought of these electrons as particles. Each particle has a specific mass, is in a specific position, and moves along in a certain, specified way.
But with quantum physics, there’s nothing so certain and fixed. Because of being so small, we can’t really see where subatomic particles are(I’ll explain why), so for us, they exist in clouds (a place where their existence probability is the highest) instead of a fixed place.
Cause of them not really being particles(For us), Quantum physicists instead of assuming them as particles and doing mathematical functions on them, describe these subatomic particles using waves. So instead of a fixed place where an electron might be, the position of an electron looks something like this —
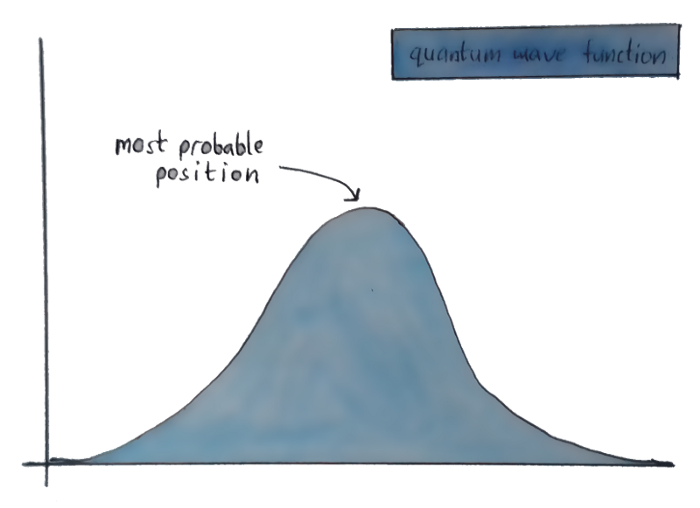
You don’t know where exactly the electron is, but the graph shows the different places it could be, and which of those places you’re most likely to actually find it.
Now, just to be clear, this is not a physical wave like the ripples we see in water or like the sound waves we’re familiar with. This is just an abstract mathematical wave, that gives us a mathematical description of an electron.
This wave comes as the solution to Schrodinger’s equation, which looks something like this —
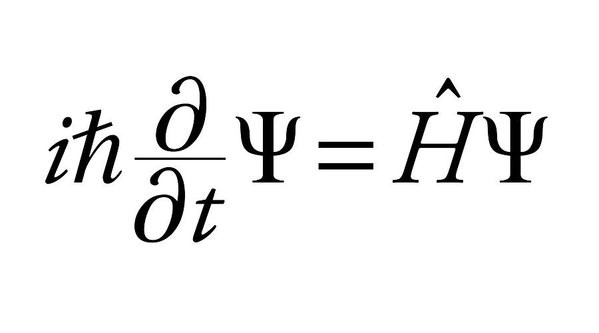
So yeah, you can see pretty clearly that it’s just math.
When you solve this equation and plot the solutions on a graph, you get the wave-function. This is very similar to the algebra you do in high school, where you solve a quadratic equation and draw its wave on the graph paper.
So if something uses wave-functions, isn’t it basically a wave? Not necessarily: the wave-function is just a graph.
Here’s another graph:
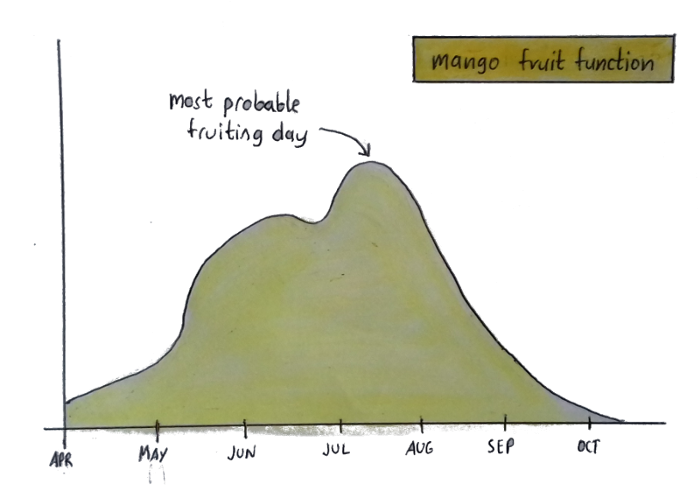
This graph tells me how likely it is for my mango tree to fruit on a given day. But that doesn’t mean my tree actually looks like a curvy line — that’s just a visual depiction of when you can expect fruit.
In physics, when people say something “is a wave”, they usually mean it follows certain rules.
When the hump, or “crest”, of a wave merges with another crest, they combine to form an even bigger one. And if a crest comes in contact with a valley, or “trough”, they cancel each other out. This combining or canceling is called “interference”, and it works the same way whether you’re looking at birds causing ripples on a telegraph wire or a music melody floating through the air.
They don’t necessarily do it the same way — telegraph wires swing up and down, whereas music and other sounds are caused by compression of air — but they still do it.
Electrons are not exactly wires or music, but their wave-like equations do come in pretty handy.
Suppose you want to calculate the position or momentum of the electron, you can easily do it using the wave function.
To find the position of this electron, you take the “amplitude” of the wave function, meaning how high it is from the baseline. Then you square that and construct a new “wave” on a graph. That “wave” would give us the probability distribution of finding the electron in that particular place — which is to say, the list of places the electron could be, and how likely it is to be in each.
Remember, I said probability. In Quantum Mechanics we can’t know anything with absolute certainty. So I can’t say for sure that the electron is now at the point with the highest amplitude, but what I can say for sure is, it has the highest probability of being there.
Why is Quantum Mechanics so fuzzy and un-exact? Well, after a point, it’s literally impossible to measure things precisely. You can’t just see where an object is, and here’s why.
To see an object, we first have to look at it. That usually means you shine some light on it, and shining light means bombarding it with photons. Okay, so what’s the problem with this?
For big objects like mice and footballs, shining light doesn’t really matter. But on a smaller scale, it does. All light has energy, you see, and when you’re a tiny electron going about your business, then a big heavy photon smashing into you will surely throw you off the direction.
If you use light, you can measure where a particle was, but not where it’s going.
What if you use less light? Well, there’s a limit to how less they can get. You can’t send light in any arbitrary amount, but only in bundles of a certain amount of energy. These bundles are what are called “photons”, and you can’t have “half a photon”.
By the way, I’m calling it “bundle” here, but the actual word is “quantum”, or “quanta” if it’s many. And that, incidentally, is where “Quantum mechanics gets its name.
So you can’t know where a particle is without bumping it at least one quantum’s worth out of the direction. But what if you use a smaller quantum?
Not all light-waves have the same amount of energy. A quantum of red light has less energy than a quantum of blue because it vibrates less rapidly: its frequency is lower.
The problem is, lower-frequency light means wavelengths are much longer. So you can figure out where a particle’s going because you won’t bump it out of the direction, but your measurement as to where exactly it is will be imprecise. Longer wavelengths mean your reference points are much farther apart: it’s like having a ruler with kilometers instead of centimeters.
That’s why things are so vague. That’s why things are so uncertain. And, that’s why this thing is called Heisenberg’s Uncertainty Principle.
Werner Heisenberg, who came up with the principle, didn’t exactly think of photons and bumpings. He tried it using wave functions but came to the same conclusion.
Theoretically, you can find out where a particle is with the wave-function: just measure the amplitude. You can also find its momentum: just measure the wavelength. But imagine an electron having a random wave-function like this one —
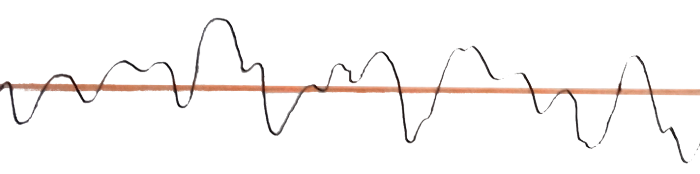
The amplitude of this wave can be calculated easily and thus, the probability of finding this electron at a particular point is known. (We don’t accurately know its position, but let’s ignore that for a moment).
Now, consider the wavelength of the wave.
Yes! exactly, this wave has no fixed wavelength, and because of this, we are not sure about its momentum.
What if you tweak the equation around to get a regular “sine wave”?
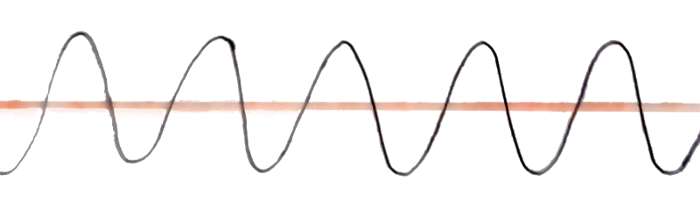
This wave has a fixed wavelength, and also it is not difficult to find its amplitude, but the problem here is that this wave doesn’t end anywhere. So the electron has an equal probability of existing anywhere at any point in the entire universe.
Using different kinds of light, you can know either where a particle is, or how it’s moving — but never both at the same time. It’s impossible to know both with absolute certainty. Even with wave-functions, you get the same result.
In a nutshell, the principle is —
“It is impossible to know simultaneously the exact position and momentum of a particle.”
The more exactly you determine the position, the less you know of its momentum — and vice versa. So at a particular time, we can’t calculate both the position and momentum of the particle.
The Uncertainty Principle had great philosophical implications. For the first time in history, physics admitted that it could never know some things accurately. That, however, is just the tip of the quantum iceberg.
So if you think Heisenberg’s idea is the weirdest in the world — well, wait till part two and perhaps you won’t be so certain.
Quanta in a Nutshell: This article is one of a four-part series on quantum mechanics. Up next: double-slits, self-conscious particles, and why duality is like an elephant
Want to write with us? To diversify our content, we’re looking out for new authors to write at Snipette. That means you! Aspiring writers: we’ll help you shape your piece. Established writers: Click here to get started.