What a hot cup of coffee tells us about the history of life on Earth
What is life? Life, the empirical phenomenon, is (to use a simple definition) a collection of molecules that are capable of self-replication, with slight mutations. More interesting, however, is to ask: why is life?
The answer may lie in thermodynamics, in the laws that govern the flow of energy.
Let’s say you have a mug of coffee sitting in a room. Heat energy is stored in that mug, in a rather distinct configuration.
(In the universe, collections of heat energy in the particular shape of that coffee mug are bound to be quite rare).
The Second Law of Thermodynamics tells us that energy will flow, out from the mug and into the room, to make this configuration less distinct. It will try to organise itself in a form that’s more common, random, or disorderly. To use the scientific term: from low entropy to high entropy.
When energy flows, it enters molecules and atoms, exciting them and sometimes causing them to enter new — and again, distinct — configurations. This might seem strange: after all, isn’t energy only flowing because it doesn’t want to remain in distinct configurations? It’s a bit of a paradox, but these small instances of increasing distinctness or order actually help increase the system’s overall randomness, “boringness”, or disorder.
They’ve absorbed energy which was all concentrated in a mug, and are now bouncing around, touching other molecules, spreading the energy far and wide. Thus, they’re restoring the entire room to the less distinct, more evenly-spread-out configuration of energy it was in before you introduced the coffee mug.
This brings us back to the question we started with: why is life? Consider an undersea vent a few billion years ago. Energy is flowing from molten lava into frigid water. There’s a high “gradient”, or difference, between these two. Thermodynamics tells us that energy must flow to reduce the gradient and make the configuration of energy more random.

As molecules around the lava are excited by this flow of energy, they begin to join together into orderly little groups of molecules, which would bounce around, joining up with other groups to form new ones. By moving around in chaotic ways and impart that energy to more molecules, these orderly little groups actually help increase the overall disorder of the entire system.
As they agglomerate, these groups of molecules develop all sorts of random chemical properties. At some point, some groups develop the property to feed off that energy gradient and make copies of themselves.
Let’s say that we have two such groups, both of which are capable of self-replication. One group — let’s call it Super-Molecule A — is perfect at it. Every replica is exactly the same as its “parent”. The other — Super-Molecule B- is somewhat imperfect. Every replica is slightly different than its “parent”. Which one survives and thrives?
The answer, again, might be counter-intuitive — it’s the second one.
Why does the imperfect B survive and thrive better than the perfect A? Because the descendants of the first group can’t get better at processing energy. They’re all only as good as the initial template. Whereas the second, through random reconfigurations, creates a whole bunch of copies, some of which are worse at processing gradients — and some of which are better. The ones that are worse make less and less copies of themselves. The ones that are better process more gradients and create more copies of themselves, some of them worse, some of them even better. The effect snowballs across generations. B outbreeds A, and becomes the ancestor of life.
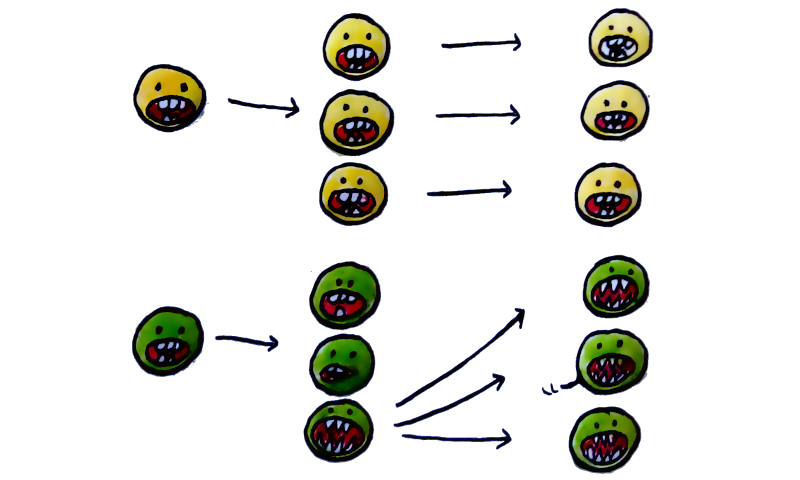
An orderly little machine, processing an energy gradient to make slightly different replicas of itself, and thus increasing the overall disorder in the system’s distribution of energy.
Of course, life didn’t stay confined to waters around volcanic vents. The little low-entropy machines, constantly seeking to feed off energy gradients, spread out across the world into a plethora of different environments, all with different levels of energy available.
They had to. How else would the trapped and bunched-up energy get evenly spread throughout the Universe?
Entropy is fundamentally about equilibrium: about things being equal. It all goes back to the Big Bang.
“Before” the Big Bang, energy and matter were all contained in one infinitesimal point. Time and the fundamental forces of nature didn’t exist. Order and disorder had no meaning, energy and matter saw eye to eye, and everything was happy and equal.
Then came the Beginning. The Bang.
Nobody knows what exactly happened — but once it was over, all these concepts decoupled from each other. Time and space emerged to become the fabric upon which gravity and the atomic forces played their great game, creating new elements in the furnaces of new stars and ordering the cosmos into galaxies and superclusters.
And what was — is — the purpose of the game? It’s actually very simple: to return to that state of perfect equilibrium until space and time, order and disorder, energy and matter have just as much, or just as little, meaning as they did when they started out.
There were two ways this could happen. The laws of physics could have mandated that things stick together, becoming more orderly over time until they were infinitely ordered. Instead, our Universe mandates that everything drifts apart, becoming more random over time until they are infinitely disordered.
This is the only reason that energy — and even, for that matter, matter — obeys the Second Law of Thermodynamics. There were many possible paths the Universe could have taken in establishing its post-Bang physics, and this is the one it chose.
As you can see, it takes something very, very special to apparently defy this fundamental law.
In each environment, the “special somethings”, those little machines, that were life, diversified further. They became more complex and orderly, becoming highly specialised types of machines that could process distinct “slices” of energy available in a given environment. Solar, energy stored in other machines, anything: it didn’t matter as long as it was energy.
Types that were good at it made more versions of themselves. Types that weren’t died off.
Over hundreds of millions of years, this drive to mutate, to increase internal complexity in order to increase overall randomness in the distribution of energy, created incredibly complex machines.
From insensate little creatures, they mutated over generations into forms that could move, developed little photosensitive patches that could sense photovoltaic and chemical variations in the environment around them, grew larger, and figured out how to transfer information within their bodies and coordinate responses.
They learned how to eat other machines and how to escape being eaten by other machines. They began to evolve in response not just to energy-environments around them, but in response to energy-environments now shaped by other machines. Complex systems of interactions developed, that we now call “ecosystems”.
The machines called plants processed solar energy. Herbivores processed plants. Carnivores processed herbivores. And so on.
Fish. Amphibians. Dinosaurs. Mammals.
Every now and then, changes in energy distributions sparked off the emergence of radically new machines that were better suited to doing their task. Massive volcanic events blotted out solar energy, cascading through ecosystem networks and killing off many species. Continents drifted, stranding some machines in their own distinct energy environments that allowed for even more diversification and specialisation. New evolutionary pressures incentivised the emergence of new forms of life.
Eventually, a mutation in one rather insignificant machine gave it the ability to process language. That was the ancestor of Homo Sapiens.
Language was a revolution. All of a sudden, human life-machines could interact to a far greater degree of sophistication than ever before, leading to the emergence of more complex networks.
These social networks enabled them to coordinate to hunt and take down much larger machines, from trees to mammoths. Humans were now flexible enough to harness a much more massive range of energy sources.
The result? More self-replication, more entropy. A population explosion. Migration. Large-scale ecological change.
Human social networks were organised in a plethora of different ways. But one, in particular, proved to be especially good at enabling them to harness and redistribute energy: The State. States that were better at processing energy and increasing entropy could become more and more orderly and complex and better at doing that (paradoxical, I know). Other social structures that weren’t up to the mark were simply gobbled up by states.
Within states, human functions further specialised. By connecting a vast number of humans together, states allowed the emergence of “professions”. By enforcing trust in an imaginary concept called “money”, states allowed specialised humans to interact in increasingly complex ways. Cultures and civilisations emerged on the fringes of the deep eternal drive to process energy gradients and increase entropy.
This process is what we call “history”.
States weren’t invincible, of course. They had their own sets of vulnerabilities. Vast empires could be shattered by a slight change in solar energy levels, or the emergence of a rapidly-spreading biological machine (like a plague) which destroyed information and energy redistribution networks and thus rendered their degree of complexity unsustainable.
Consider the Roman Empire. A fall in insolation levels in the 3rd century CE made agriculture less sustainable, which cascaded up various human networks in chaotic ways, causing famines and wars, eventually leading to a “mass extinction” of social complexity similar, in principle, to mass extinctions of biological complexity that the Earth has seen countless times.
Eventually, though, humans evolved social networks that were sufficiently complex to import energy from other parts of the world, and extract it from the Earth itself. That done, states became practically impervious to environmental redistributions of energy (such as volcanic eruptions). The result? Exponentially increasing social complexity and order. Ever-increasing entropy.
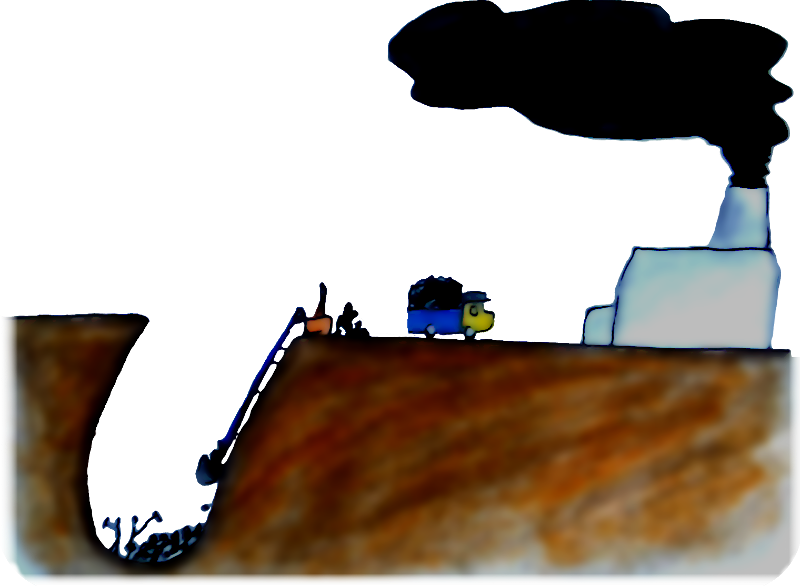
An example: what business does energy have being stored in coal and oil? Humans evolved organisational structures that were complex and orderly, extracted the energy, and turned it into spinning tyres and puffing exhaust pipes — a more random and high-entropy configuration.
Modern human networks are incredibly complex and interconnected. They are vastly more resilient to environmental change, as mentioned. Yet they are also changing the environment to a much vaster degree than ever before. Chopping forests can lead to the introduction of new diseases that were hitherto confined to animal species that we rarely interacted with, tearing into delicate human organisational structures and bodies, leading to the global COVID-19 pandemic we are struggling with as I write this.
Or, in the near future, global environment that is inhospitable to (for example) plants, which process solar energy into forms that sustain other life forms, will cascade through now densely interconnected human networks, causing famines, wars, and mass migrations, and cause an extinction of social complexity that will make the collapse of the Roman Empire seem like a child kicking over a sandcastle.
The solution? Change our social and economic systems so they damage the environment less. But these highly-ordered systems are the basis of human existence as we know it — to change them will require an unprecedented global effort that leads to a totally different way of life. Whether we will do so remains to be seen.
But not all hope is lost for all human life-machines, not yet. We could save ourselves.
Or not. The universe doesn’t care. Energy gradients must not persist. Entropy must increase.
And it will — whether you like it or not.
