What really happens when you charge your battery faster?
Visiting New York City has always been a dream of mine, and, for the first time in my life, I had travelled there by flying out from Seoul. It was loud and bright and an absolute maze. Fortunately, I had my trusty Google Maps!
At the heart of Manhattan, I was running around the places where I planned to visit and tour, but realized my cell phone battery was running low. I had many more places that I wanted to look around, but I did not even figure out where I am. To the complete stranger in NYC, it was a maze. Without Google Maps, I would be completely lost and would lose my priceless vacation time.
Frantically, I began looking for a convenience store to use a fast charger. I eventually found one at a 7-eleven plugged it in, and, to my relief, the phone was fully topped up in just around ten minutes.
It was only later that I realized fast-charging increases the failure rate, and eventually reduces the life of the battery. Fast chargers are certainly useful in emergencies, but I should have avoided it as much as I could.
You could say the modern world runs on lithium-ion batteries. Lithium-ion batteries are everywhere. In your portable devices such as cell phones, tablets, laptops, video cameras, and all kinds of wireless electronics around you.
Recently, you have begun to ride lithium-ion batteries beyond carrying in your pocket and backpack. I’m talking about electric vehicles from the commercialization of full-electric automobiles such as Tesla Model 3. Not only Tesla, but almost all automobile makers have been preparing to sell fully battery-operated cars and trucks. As mentioned, we have carried and ridden lithium-ion batteries, and then we try to build a lithium-ion battery farm to provide residential and commercial buildings with electricity in utility-scale.
Depending on your location, around 30–40% of the electricity we are using daily already is from lithium-ion batteries. Sometimes we see lithium polymer batteries, but the basic chemistry is the same.
And, of course, the fast charging/discharging problems are the same as well.
Now here is the thing with batteries: they need to be charged. You need to keep them topped up every night to make sure they last you the next day — or, if you are lucky, the next few days or even a week. You battle a clump of tangled wires, painstakingly picking out the one you need. You disconnect other devices to free up sockets, in a move that will come to haunt you later when your headset runs out of charge or your e-book reader freezes on its page.
Because of this, you may be glad that some companies provide you with “super” or “fast” chargers. They get your charger filled and unplugged at a rapid rate, no questions asked. (Of course, charging speed is probably one thing with which we will never be satisfied. Not unless they charge your device the second you plug them in).
However, I would not recommend charging faster than for a one-hour finish, if possible and comfortable. Batteries are designed for slower, one- to four-hour charging sessions, and with good reason.
Overloading and fast discharging can never be fruitful for batteries. Fast charging? Even less so.
How does a lithium-ion battery work, exactly? The word ‘ion’ provides a clue. An ion is basically a ‘charged’ atom. Lithium-ion batteries use lithium ions as ‘charge carriers’, to store and utilize energy.
In batteries, the ions shuttle between two endpoints; the ‘cathode’ and the ‘anode’ as they say. When you use your battery, the lithium ions run from the negative electrode (anode) to the positive electrode (cathode) — and, because they’re going from a ‘more energy’ zone to a ‘less energy’ one, they release some energy in the process.
In a fully discharged state of the battery, all the lithium ions eventually end up at the cathode. There’d be no more energy to release, and that would be the end of the story. So how would rechargeable batteries work?
You guessed it — they have to somehow ‘charge’ themselves by moving lithium ions back from the cathode to the anode.
‘Charging’ a battery sends an electric current running through it, which pushes the ions away from the cathode and back to the anode. In lithium-ion batteries, graphite has been the standard anode material for a long time. Lithium ions are embedded into the graphite, and slowly come out when it’s time to discharge. In this case, the graphite anode is able to operate as fast as the cathode does, releasing ions on one end as they’re absorbed on the other.
But it doesn’t work that easy the other way round.
When you charge your battery, lithium ions zip out of the cathode to penetrate the anode’s carbon layers and embed themselves in their midst. But the graphite anode can’t accommodate them fast enough. Instead of going in and settling down, the ions pile up on the surface in one big traffic jam.
Over time, this pileup of ions causes growth on the surface, something like the stalactites and stalagmites you find in caves. These growths, however, are known as “lithium dendrites”. (And, they grow sideways instead of up and down).
Repeated dendrite formation is a problem, because parts of these dendrites break off and become dead lithium particles. These particles are not connected to anything. Detached from the anode, they can no longer take part in the electrochemical reactions to store electricity, and simply float around like zombie particles.
As you can guess, the amount of lithium in a battery cell is limited. There’s only as much as the cathode originally contained at the time of manufacturing, before it was pulled back and forth through charging and discharging. So when there’s dead lithium floating around, there’s obviously less ‘live’ lithium to actually do the work.
In other words, the lifetime of the battery goes dramatically down.
Maintaining a stable battery operation helps to sustain battery health. In other words: don’t charge or discharge too fast, because then the ion absorption will go out of sync and cause a pileup. The more you care for your batteries, the less pileup there will be, and the longer your battery will last.
But that’s not the only problem.
Remember the dendrites that form from the pileup? Just like the stalactites and stalagmites in a cave, they keep on growing. And unlike caves, batteries can be short-circuited.
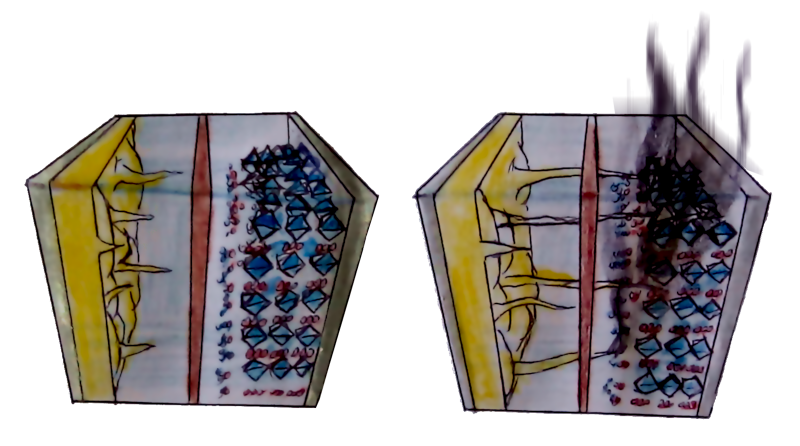
When sharp fingers of lithium dendrites reach the other end of the battery cell, they could induce a short circuit inside. This means not the only failure of the battery, because you’ll immediately lose 100% of the energy in the cell, but it could also cause a fire due to elevated temperature and flammable electrolytes.
Of course, battery manufacturers recognize these issues and have already considered them in the development process.
A tremendous number of engineers and scientists are working on fixing this, and there’s no doubt the technology has advanced remarkably. From these efforts, the risks of shortening lifetime and fire hazard might be minimized as much as we can. Until then, however, we can substantially decrease these kinds of failures by using batteries at slower charging speeds as much as our situations allow.
Don’t dispose of your battery in flames. That’s obvious. What you should remember is that overly sped-up charging has exactly the same effect.