The science and history behind the making of butter
I distinctly remember the history unit in fourth grade that taught us about American pioneers and the Westward Expansion for the first time. The unit culminated with all the fourth graders dressing up in old fashioned garb and going on a field trip to a historic site called the Old Wade House.
During the tour, the guides wanted to demonstrate how the pioneers used a butter churn to turn milk into butter. Thinking the churn looked fun, I volunteered to help make the butter, but was I in for a reality check.
Within minutes, the plunger was feeling heavy in my hands and the liquid in the churn still resembled milk!
For the first time in my young life, I appreciated the convenience of the food around me. And it may just be this appreciation that drove me to major in food science many years later.
Long before we ate croissants or bread and butter pudding, an Indian deity — Lord Krishna — was stealing butter as a little boy. The legends of Krishna’s childhood and youth describe him as a mischievous young boy who (of course) plays the flute and charms all who knew him.
Young Krishna, who was very fond of butter, would rope his friends into helping him steal it. The story goes that he would wait until the clay pots that contained butter — which were hung from the ceiling — were left unguarded in the storage room, and then quick as a mouse climb up on the backs and shoulders of his friends to break open the handis (the pots). Some folktales even say that Krishna would crawl up rice sacks and drums as just a mere toddler to get his favourite food.
Today, in parts of India, Hindu festivals retell the story of Lord Krishna and the butter by forming human pyramids to break open handis — which are hung high in the air — and spill it all over crowds.
As you can see, butter has a long and vibrant history; it’s a part of cultures all over the world, but one thing that remains the same is the process of converting cream into a buttery spread.
While most of us love butter, and use it in our cooking, do you have any idea how butter is made? Sure, maybe you know we start with cream, but how do we turn this liquid into something more solid?
The first clue to this transformation can be seen by zooming into cream to better understand its structure. Although the details of the cream would be near impossible to see with the naked eye, a microscope would reveal tiny oil droplets in water. Without further ado, here’s our first close-up view of cream:
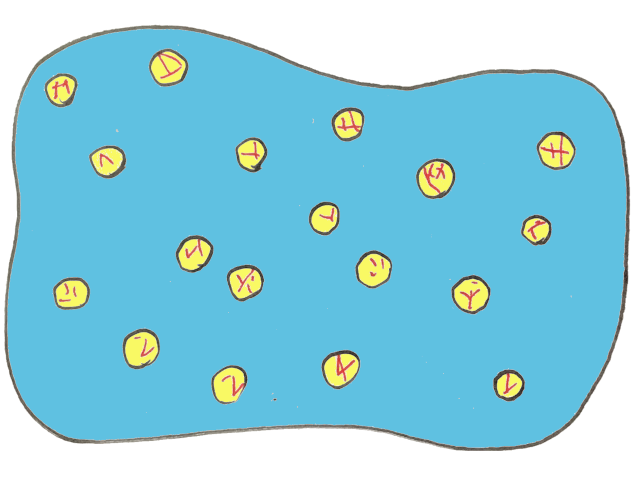
Notice the little droplets of oil floating about inside? Anytime you have droplets of one liquid (oil, in this case) dispersed within a second liquid (water), we call this food an ‘emulsion’.
Emulsions are any foods that are made up of two liquids that don’t mix — like oil and water — where the first liquid is small droplets held within the second liquid. The droplets are typically referred to as the dispersed phase whereas the liquid that encompasses the droplets is known as the continuous phase.
Since cream has oil droplets held within a watery, continuous phase it’s termed an oil-in-water emulsion.
What’s peculiar is that butter is also an emulsion, but a water-in-oil emulsion. Meaning the water has somehow been turned into discrete droplets and the oil now encompasses the water droplets. This switch is called phase inversion.
How’s this possible? How do the phases get switched or inverted? To understand that, we need to follow the structure of cream as it slowly develops into butter.
If you look closely at the picture of cream you’ll notice little lines or spears within the oil droplets. Here some of the oil has crystallized into solid fat, meaning the droplet is mostly liquid oil but also contains some fat crystals.
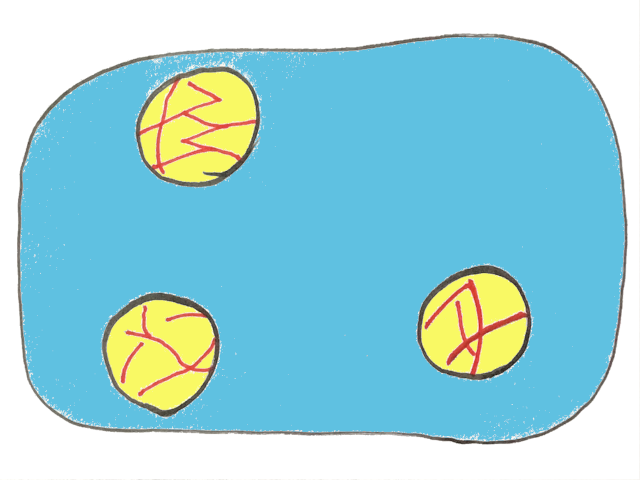
This seems like an insignificant detail right now, but it’ll be much more important later, so keep it in mind!
As mentioned earlier, butter is not a recent discovery — it is as old as time.
It’s believed that the word butter is derived from the Greek word bouturon which means cow cheese. However, the origin of the word is debated since the Greeks had mostly sheep and goat milk which was relatively low in butter fat.
The first occurrence of butter is thought to be by complete accident. It’s likely that a nomad was simply trying to store his milk in a sheepskin bag on one of his pack animals, but it ended up churning into butter. From there, each culture developed slight variations of this butter making technique.
One of the oldest known methods was used by the Arabs and Syrians and similar to the nomad’s method. They used goatskin as a vessel to churn!
A goatskin was tightly sewn up to form a bag that had an opening near the left foreleg for the cream to be poured in. The “churn” or bag was then hung from tent poles and swung until the butter was formed!
The natives of Mexico are known for loading a bag of milk onto a horse before taking a journey. That way all the churning would be done before their arrival at their destination.
The ancient Irish had a unique ageing step where they buried barrels of butter into bogs. The cool temperatures of the bog would have prevented the butter from rapidly spoiling.
Finally, we can get into how butter is made, which unlike older methods will require no wild animals!
Believe it or not, it’s so easy you can do it at home using supplies found around the house.
To begin with, find any small to medium container with a lid. I’ve used mason jars and Tupperware containers in the past.
Next, fill up the container about halfway to three-fourths full with cream. You want to leave enough room to agitate and shake the cream up and down in the container.
Finally, get those arms muscles ready for a workout because you’re about to shake this container for 5–10 minutes, or until you make butter.
Going back to our earlier method; when you first start shaking the cream, what you’re doing to the structure is incorporating tiny air bubbles. And you can see after 1–2 minutes of shaking that container that you have whipped cream. It looks like something you might scoop onto your dessert.
And incorporating these air bubbles is really important to start the process of making butter because the oil droplets will make their way to the air bubble.
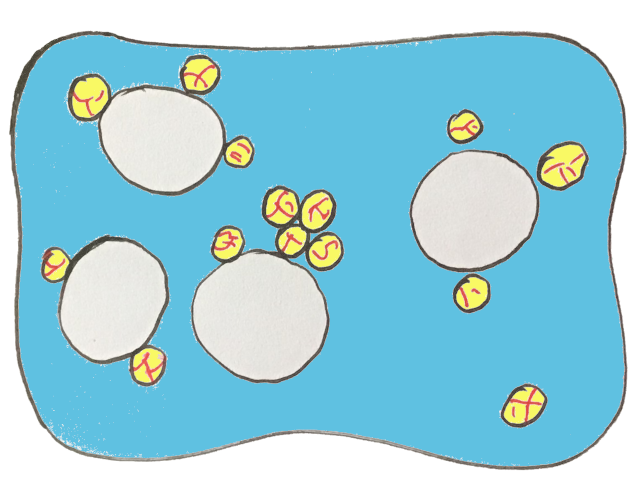
Why does this happen? Well, oil and water don’t like each other, so given a choice, the oil droplets choose to be by the bubble of air instead. You’ve probably heard the old adage: oil and water don’t mix.
If you’ve ever blown bubbles as a kid, you know they don’t last long. The same is true for the air bubbles in the cream — they start to pop.
And as these bubbles collapse, the oil droplets that have been surrounding them are suddenly brought together. The oil in the droplets acts like glue, allowing the droplets to get pulled towards each other, forming a larger cluster of oil droplets.
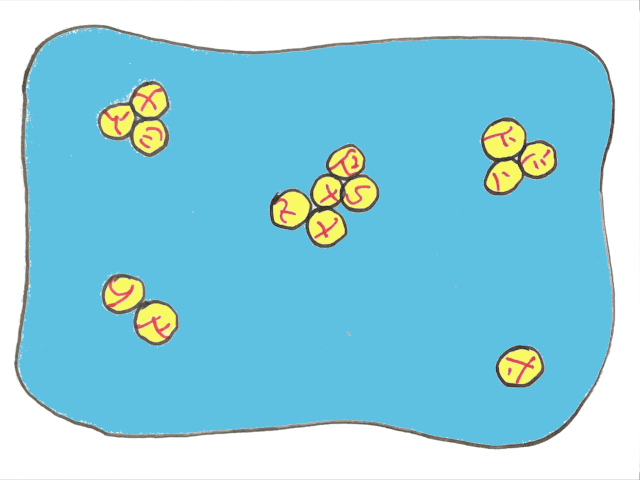
There’s a special term for when oil droplets only partly merge together — partial coalescence. And the reason they can only partially combine is the solid fat within the liquid oil stops the droplets from fully merging.
This is why the presence of solid fat crystals is so important. If you want to make butter, the cream needs to have some solid fat within the oil droplets to get partial coalescence.
Initially, the collapse of the air bubbles allows the oil droplets to come into contact and produce clusters. After these initial fat clumps are made, it’s enough to simply continue agitating the container to force more and more oil droplets to collide with the clusters. The goal here is to grow those initial fat clusters to larger sizes.
We’re not quite at butter yet but we are getting close.
As the fat clusters grow larger and larger, they begin to collide with one another. With a little more agitation, the clusters soon combine to form one large fat network. This is where phase inversion happens.
The fat network is so strong it can hold in water droplets. Water is now the ‘dispersed’ phase and the oil (and fat) are the ‘continuous’ phase. So the situation is exactly the same as before…but the other way round!
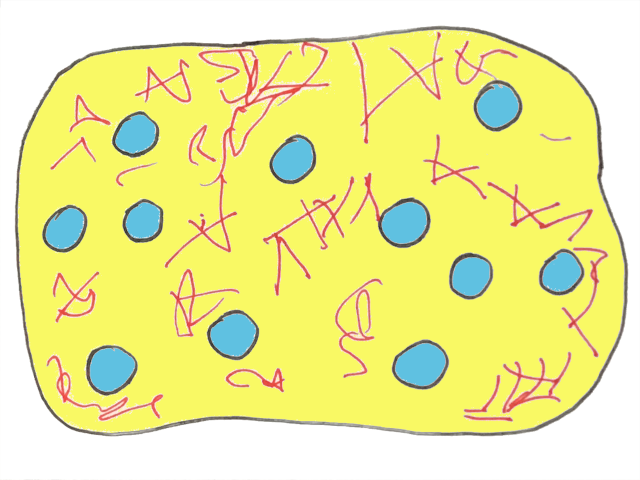
Finally, butter at last.
Any food like butter (a combination of oil and water) is prone to separation. Luckily, the solid fat crystals — that were once held within the oil droplets and are now distributed throughout the continuous oil phase — prevent the water droplets from moving around. It’s really these crystals that stabilize the water-in-oil structure of butter and allow it to sit in your refrigerator for weeks.
Whether it be a young Krishna or a child in your life, I think we can all understand the urge to sneak some butter for a savoury snack. There is something about that buttery goodness that’s just so difficult to resist.
The good news is, butter can also be a great example of science in real life. So let that child have their buttery treat, but also teach them about the scientific transformations that are needed to make this delicious food. And if you have the time, spin a tale or two about the Indian god, the ancient Irish, and other butter stories from around the world!